تاریخ بروزرسانی(Update Date): 3rd February 2025
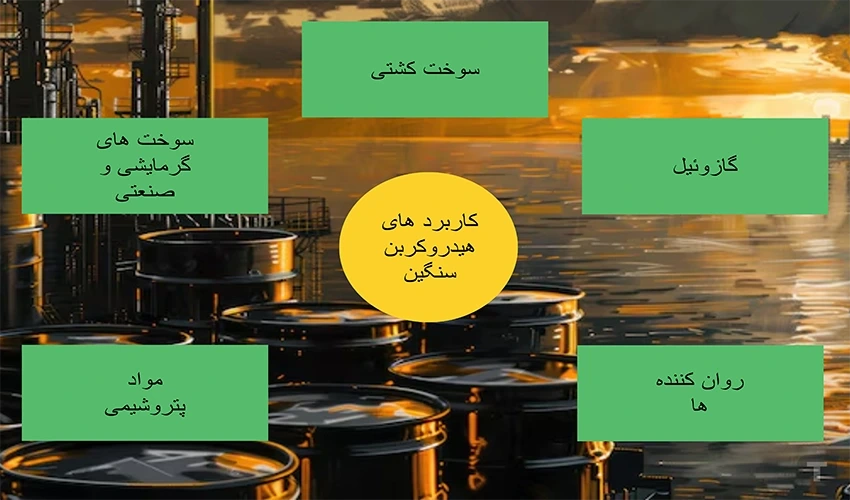
Application of Heavy Hydrocarbons
Heavy hydrocarbons, as one of the key components of crude oil, play a vital role in various industries, especially refineries and petrochemical plants. These compounds, due to their large molecular structure and physical properties different from light hydrocarbons, are used for producing a variety of products after conducting a thorough analysis of heavy hydrocarbons. The analysis of heavy hydrocarbons helps determine their chemical composition and physical characteristics, enabling their optimal use in various processes. Among these products are marine fuels (Bunker Fuel), lubricants, and raw materials for certain petrochemical processes. In this article, we will explore the application of heavy hydrocarbons in different industries.
Bunker Fuel Production

A significant portion of marine fuel is produced from heavy hydrocarbons, although it should be noted that some ships operate on alternative fuels such as LNG (liquefied natural gas) and biofuels. The production of marine fuel requires adherence to strict standards and high precision. These fuels must be carefully adjusted in terms of density, viscosity, and sulfur content to ensure optimal performance of marine engines and reduce emissions.
Diesel Production
Diesel is produced from a portion of heavy hydrocarbons. After the crude oil distillation process, the heavy hydrocarbons that accumulate at the bottom of the distillation column are separated. These hydrocarbons then undergo two key processes: cracking and hydrotreating.
In the cracking process, also known as “molecular cracking,” larger heavy hydrocarbon molecules are broken down into smaller and lighter molecules. This process can be carried out either thermally or catalytically, with the goal of producing fuels such as diesel with a more suitable molecular structure. This is an example of how heavy hydrocarbons are utilized to produce cleaner and more efficient fuels.
Next, the hydrotreating process is used to improve the quality of the diesel by reducing sulfur and other impurities. This process ensures that the produced diesel is cleaner and meets modern environmental standards.
Lubricant Production

Lubricants, which are used to reduce wear and friction between closely moving parts in equipment, play a vital role in preserving the lifespan and efficiency of these machines. Many lubricants are produced from heavy hydrocarbons. The application of heavy hydrocarbons in lubricant production occurs through various stages.
After the separation of heavy hydrocarbons from the bottom of the distillation column, these materials are sent to further processes, including hydrotreating and cracking. In the hydrotreating process, impurities such as sulfur and nitrogen are removed from the heavy hydrocarbons to improve the quality and stability of the base oil. Additionally, in some cases, cracking is used to break larger molecules into smaller, more suitable ones for lubricant production.
The result of these processes is the production of high-quality base oils, which serve as the raw material for industrial and automotive lubricants. These oils possess features such as high thermal stability, oxidation resistance, and friction reduction, making them highly effective in various applications, from automotive engines to heavy machinery.
Petrochemical Production
After undergoing cracking and hydrotreating processes, heavy hydrocarbons are converted into raw materials used in the petrochemical industry for producing polymers and other chemical products. These processes break down the molecular structure of heavy hydrocarbons into lighter and more useful compounds that serve as feedstock for petrochemical units. Below are some applications of heavy hydrocarbons in the petrochemical industry:
- Ethylene – for the production of plastics and polymers.
- Propylene – used in the manufacturing of polypropylene and polymeric materials.
- Butadiene – for the production of synthetic rubber.
- Benzene – used in the production of polystyrene and nylon.
- Toluene – for producing solvents and other chemicals.
- Xylenes – used in the production of polyester and PET.
- Polyethylene – for making packaging and pipes.
- Polypropylene – used in the production of plastic parts and synthetic fibers.
- Polystyrene – used in the production of packaging materials and foam.
- Polyethylene Terephthalate (PET) – for manufacturing plastic bottles and fibers.
- Alkylbenzenes – used in the production of detergents.
- Synthetic Rubbers – for making tires and rubber products.
Industrial and Heating Fuels
The production of industrial and heating fuels from heavy hydrocarbons is one of the key applications for supplying energy in large-scale industrial processes and heating systems.
One of the largest consumers of heavy hydrocarbons is power plants. These plants use heavy fuel oil to generate steam, which in turn drives electricity-generating turbines. The high energy density of these fuels allows for a significant amount of energy to be produced with relatively small amounts of fuel.
The cement and steel industries are also major consumers of heavy hydrocarbons. These industries require extremely high temperatures in their furnaces to carry out processes like metal smelting and clinker production for cement.
In industrial heating systems, heavy hydrocarbon fuels are used to provide the necessary heat for large spaces such as factories, warehouses, and commercial buildings.
Outlook for Heavy Hydrocarbon Applications
The outlook for the application of heavy hydrocarbons is complex and influenced by factors such as technological advancements, environmental pressures, and changes in energy markets. While these resources remain essential for certain industries and producers of both light and heavy hydrocarbons, the growing focus on renewable energy and reducing greenhouse gas emissions is likely to lead to a gradual decline in the use of heavy hydrocarbons. However, smart utilization of modern technologies and proper management of these resources can help optimize their use and minimize their negative impacts.